Knowledge Base
Immunotherapy: What Neurosurgeons Need To Know
By: Maryam Rahman MD, MS, FAANS
Assistant Professor, Department of Neurosurgery
Preston A. Wells Jr Center for Brain Tumor Therapy, University of Florida
Cancer immunotherapy was declared “Breakthrough of the Year” in the journal Science in 2013. Various immunotherapeutic strategies have had remarkable results in cancers such as melanoma, lung, renal and prostate cancers. These findings have lead to increasing interest in using immunotherapy for brain and CNS tumors. The underlying mechanism of all immunotherapeutic approaches to cancer relies on cytotoxic T cell recognition of tumor cells to allow for tumor cell death. Use of immunotherapy for brain tumors has had notable successes and disappointments. The immunotherapy strategies available will be reviewed here as well as a brief review of important immunotherapy clinical trials for brain tumors.
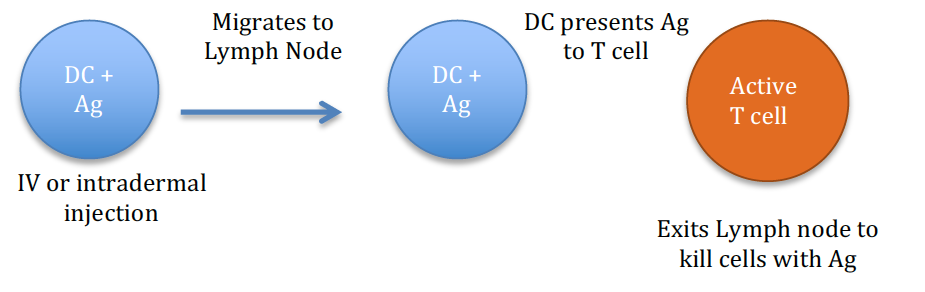 Vaccines:
Vaccines:
Cancer vaccines are dependent on the function of dendritic cells (DCs) which are professional antigen presenting cells (APCs). The cancer vaccine delivers an antigen typically to an intradermal site where DCs can take up the antigen (Ag), migrate to the draining lymph nodes and then present the antigen to T cells. If the T cells recognize the antigen as foreign, they will mount a robust immune response. CD4 helper T cells will release cytokines to allow for proliferation and maturation of CD8 cytotoxic T cells. The cytotoxic T cells then exit the lymph node. If they encounter the antigen or cells containing the antigen, they will cause lysis of the abnormal cell.
Another similar approach is the use of an oncolytic virus used to inject directly into tumor cells. The virus causes tumor cell death and the lysis of the cells results in release of antigens and subsequently an immune response. Talimogene laherparepvec (T-VEC or Imlygic®) is an FDA approved oncolytic herpes virus that can be injected into melanoma tumors directly and also leads to regression of non-injected tumors. This phenomenon is believed to be due to the induced immune response.
The only FDA approved cancer vaccine is Sipuleucel-T (Provenge) for prostate cancer. Delivery of the vaccine requires leukapheresis of the patient. Their DCs are collected and loaded with a protein found in prostate cancer (PAP) and GM-CSF. The patients receive the final product as an IV infusion. Vaccine approaches to brain tumors include but are not limited to intradermal injections of dendritic cells loaded with tumor cell lysate, proteins such as heat shock proteins, and RNA encoding various antigens. The most well-known vaccine for brain tumors thus far is an EGFRvIII peptide vaccine (Rindopepimut, Rintego®) for glioblastoma. This was recently tested in a phase III RCT that was discontinued in March 2016 as the control arm and treatment arm had similar overall survival (21.1 mo versus 20.4 mo).
Adoptive Cell Therapy
Adoptive cell therapy (ACT) is a strategy that directly provides antigen-specific T cells to the patient. This approach is a passive form of immunity, as opposed to vaccination which induces active immunity in the host. ACT requires leukapheresis of patients, maturation and proliferation of T cells that are primed against a target antigen, followed by infusion back into the patient. Sometimes the T cell infusion is followed by vaccination with dendritic cells to maintain the antigen specific T cell population.
For cancer, the goal is to provide the patient with cytotoxic CD8 T cells that can cause killing of tumor cells. These cells can be obtained from leukapheresis or from the tumor (tumor infiltrating lymphocytes or TILs). The antigen used to prime the cells can vary from peptides to RNA or other cellular components. ACT has the versatility of being able to engineer T cells for enhanced tumor targeting, enhanced T cell survival and increased specificity. Recently, manipulating T cells to express chimeric antigen receptors (CARs) has lead to increased specificity and potency of ACT. CAR T cell therapy has been mostly studied in melanoma and hematologic malignancies. Potential side effects include cytokine release syndrome, B cell aplasia (due to cross-reactivity with antigens on B cells), and tumor lysis syndrome.
ACT is currently only available in the setting of a clinical trial.
Immune Checkpoint Blockade
Immune checkpoint inhibitors have had the most success of any immunotherapeutic strategy in gaining FDA approval for cancer. These drugs work by blocking signals that tumor cells use to reduce activation of cytotoxic CD8 T cells. There are several mechanisms and receptors that turn off T cell activation to maintain immune homeostasis. These signals are leveraged by tumor cells to evade immune recognition. These receptors include PD-1, CTLA-4, IDO, TIGIT and others. Immune checkpoint inhibitors are monoclonal antibodies that occupy either the T cell receptor or the tumor ligand to prevent interaction between the tumor ligand and T cell receptor. By blocking this interaction, the T cell remains activated against the tumor cell and can cause tumor killing.
These drugs have had the most success in melanoma, non small cell lung cancer and renal cell carcinoma. FDA approved drugs include PD-1 inhibitors [Pembrolizumab (Keytruda®), Nivolumab (Opdivo®)], PD-L1 inhibitors [Atezolizumab (Tecentriq®)], and CTLA-4 inhibitors [Ipilimumab (Yervoy®)]. Ongoing trials are being performed for primary brain tumors. Several trials have shown efficacy of these drugs in the treatment of brain metastases as well. Side-effects are mostly inflammatory reactions that can result in dermatitis, endocrinopathies, pneumonitis, etc.
Immunomodulation
Immunomodulation focuses on priming the host immune system to improve immune response or result in synergy with other immunotherapeutic approaches. These strategies include using radiation or chemotherapy to induce immunogenic cell death that releases damage associated molecular patterns (DAMPs) on the cell surface that serve as a signal for phagocytosis. This process results in stimulation and optimal antigen presentation by antigen presenting cells (APCs) to T cells which results in further killing of tumor cells by activated T cells. Other immunomodulation agents include cytokines, thermoablation, oncolytic viruses, and techniques to reduce immunosuppression in the cancer microenvironment. These strategies are only being investigated in the pre-clinical setting for brain tumors.
Impactful Published Clinical Trials
-
Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, N.Y.). 2015;348(6230):124-128.
-
Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189-2199.
-
Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (New York, N.Y.). 2015;348(6230):69-74.
-
Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517-2526.
-
Mitchell DA, Batich KA, Gunn MD, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366-369.
-
Kenter, G. G. et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 361, 1838–1847 (2009)
-
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010)
-
Dudley, M. E. et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002)
-
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011)
-
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010)
-
Ghiringhelli, F. et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 56, 641–648 (2007)
-
Not yet Published Results:
-
Rindopepimut from Celldex in ACT IV study demonstrated no survival advantage in phase III trial in newly diagnosed GBM patients comparing standard therapy plus EGFRvIII vaccine versus standard therapy plus placebo vaccine.
-
Opdivo from Bristol-Myers-Squibb Co. in CheckMate-143 study demonstrated no survival advantage in phase III trial in recurrent GBM patients comparing nivolumab (PD-1 inhibitor) to bevacizumab.
-